Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Balois-Oguchi, M. V.*; Hayazawa, Norihiko*; Yasuda, Satoshi; Ikeda, Katsuyoshi*; Nguyen, T. Q.*; Esca o, M. C.*; Tanaka, Takuo*
o, M. C.*; Tanaka, Takuo*
Journal of Physical Chemistry C, 127(12), p.5982 - 5990, 2023/03
Times Cited Count:2 Percentile:66.84(Chemistry, Physical)Micrometer-sized wrinkles in graphene are known to affect the electronic properties of graphene due to their shape and the strain variations they create. Here, we analyze the strain distribution and doping of a graphene wrinkle having 1.9 nm width using tip-enhanced Raman spectroscopy (TERS) in ambient conditions. We found a strong correlation between the TERS images of the graphene wrinkle and the electronic Raman scattering (eRS) of the Au(111) substrate. Our work demonstrates that the as-fabricated physical and electronic properties of nanometer-sized features, such as wrinkles, can be probed and studied in detail with TERS which is essential for nanodevice characterization.

Klotz, S.*; Baptiste, B.*; Hattori, Takanori; Feng, S. M.*; Jin, Ch.*; B neut, K.*; Guigner, J. M.*; Est
neut, K.*; Guigner, J. M.*; Est ve, I.*
ve, I.*
Carbon, 185, p.491 - 500, 2021/11
Times Cited Count:1 Percentile:6.01(Chemistry, Physical)Carbon disulphide (CS ) is one of the simplest molecular systems made of double covalent bonds. Under high pressure, the molecular structure is expected to break up to form extended crystalline or polymeric solids. Here we show that by compression at 300 K to approximately
) is one of the simplest molecular systems made of double covalent bonds. Under high pressure, the molecular structure is expected to break up to form extended crystalline or polymeric solids. Here we show that by compression at 300 K to approximately  10 GPa using large-volume high pressure techniques, an instantaneous reaction leads to a mixture of pure sulphur and a well-defined compound with stoichiometry close to C
10 GPa using large-volume high pressure techniques, an instantaneous reaction leads to a mixture of pure sulphur and a well-defined compound with stoichiometry close to C S which can be recovered to ambient pressure. We present neutron and X-ray diffraction as well as Raman data which show that this material consists of sulphur bonded to sp
S which can be recovered to ambient pressure. We present neutron and X-ray diffraction as well as Raman data which show that this material consists of sulphur bonded to sp graphite layers of nanometric dimensions. The compound is a semiconductor with a gap of 45 meV, as revealed by temperature dependent resistivity measurements, and annealing at temperatures above 200
graphite layers of nanometric dimensions. The compound is a semiconductor with a gap of 45 meV, as revealed by temperature dependent resistivity measurements, and annealing at temperatures above 200 C allow to reduce its sulphur content up to C
C allow to reduce its sulphur content up to C S. Its structural and electronic properties are fundamentally different to "Bridgman black" reported from previous high pressure experiments on CS
S. Its structural and electronic properties are fundamentally different to "Bridgman black" reported from previous high pressure experiments on CS .
.
Chen, Z. Q.; Yamamoto, Shunya; Kawasuso, Atsuo; Xu, Y. H.; Sekiguchi, Takashi*
Applied Surface Science, 244(1-4), p.377 - 380, 2005/05
Times Cited Count:16 Percentile:55.73(Chemistry, Physical)Homo- and heteroepitaxial ZnO films were grown by pulsed laser deposition on single crystal ZnO substrate and Al O
O substrate, respectively. The surface roughness probed by atomic force microscope (AFM) depends strongly on the substrate, which is much larger for the heteroepitaxial layer. Doppler broadening of positron annihilation measurements show existence of defects in both of the films, with a higher concentration in the homoepitaxial film. Raman scattering measurements reveal the E2 phonon vibration mode at 437 cm
substrate, respectively. The surface roughness probed by atomic force microscope (AFM) depends strongly on the substrate, which is much larger for the heteroepitaxial layer. Doppler broadening of positron annihilation measurements show existence of defects in both of the films, with a higher concentration in the homoepitaxial film. Raman scattering measurements reveal the E2 phonon vibration mode at 437 cm , which is characteristic of the wurtzite structure. These films show strong ultraviolet (UV) emission at 3.3 eV from the cathodoluminescence measurements, which indicates good optical properties.
, which is characteristic of the wurtzite structure. These films show strong ultraviolet (UV) emission at 3.3 eV from the cathodoluminescence measurements, which indicates good optical properties.
Ogata, Atsushi*; Okamoto, Hiromi*; Kusano, Kanya*; Endo, Ichita*; Nishida, Yasushi*; Sakae, Takeji*; Arai, Masatoshi*; Nakanishi, Hiroshi*; Kondo, Kiminori*
JAERI-Tech 2002-007, 28 Pages, 2002/03
no abstracts in English
Suzuki, Satoru; ; *
JNC TN8400 2000-020, 25 Pages, 2000/04
Nature of porewater in bentonite plays important roles on the mass transport in the compacted bentonite used as a physical and chemical buffer material of the multi-barrier system in the high level radioactive waste manegement Higher activation energies of diffusion in the compacted bentonite than those in the aqueous solution is due probably to change in molecular structure of water in the porewater. The Raman spectroscopy was applied to studying the structure of porewater in bentonite at room temperature. Bentonite (Kunipia F, 98-99wt% of Na-smectite) was mixed with ion-exchanged water by water content of 75, 80, 90, 95 and 98wt% of water or with 0.5M NaCl aqueous solution by 75 and 80wt% of NaCl solution. Intensity maxima of the spectra of ion exchanged water, NaCl solution and their porewater were observed near 3200 to 3250, 3400, 3630cm . These bands can be attributed to water molecules forming stronger hydrogen bond in this manner. Ratio of intensity, 3250cm
. These bands can be attributed to water molecules forming stronger hydrogen bond in this manner. Ratio of intensity, 3250cm /3400cm
/3400cm , increased from 0.97 to 1.1 with a decrease in water content of 100wt% (water) to 75wt%. On the other hand, intensity ratio of 3400cm
, increased from 0.97 to 1.1 with a decrease in water content of 100wt% (water) to 75wt%. On the other hand, intensity ratio of 3400cm /3250cm
/3250cm of NaCl aqueous solution, 80wt%and 75wt% were 0.92, 1.2 and 1.3, respectively. Since the Raman scattering near 3250cm
of NaCl aqueous solution, 80wt%and 75wt% were 0.92, 1.2 and 1.3, respectively. Since the Raman scattering near 3250cm was attributed to water molecule forming the strongest hydrogen bonding in the three bands, those changes in intensity ratio suggests an increase in number of water molecule forming strong hydrogen bond in porewater of the bentonite. The constrained porewater possibly results in the high activation energy of diffusion in the compacted bentonite.
was attributed to water molecule forming the strongest hydrogen bonding in the three bands, those changes in intensity ratio suggests an increase in number of water molecule forming strong hydrogen bond in porewater of the bentonite. The constrained porewater possibly results in the high activation energy of diffusion in the compacted bentonite.
 edges in solid systems
edges in solid systemsYoshii, Kenji; Baba, Yuji; Sasaki, Teikichi
Photon Factory Activity Report 1997, P. 421, 1997/00
no abstracts in English
Sasaki, Teikichi; Baba, Yuji; Yoshii, Kenji; Yamamoto, Hiroyuki
Hyomen Kagaku, 17(7), p.370 - 374, 1996/07
no abstracts in English
Teraoka, Yuden; Baba, Yuji; Sasaki, Teikichi
Photon Factory Activity Report, (14), P. 422, 1996/00
no abstracts in English
 CuO
CuO (CO
(CO )
)*; Akimitsu, Jun*; Katano, Susumu; *; *
Physica C, 255, p.157 - 166, 1995/00
Times Cited Count:10 Percentile:55.08(Physics, Applied)no abstracts in English
Baba, Yuji
SR Kagaku Gijutsu Joho, 5(2-3), p.2 - 8, 1995/00
no abstracts in English
Yamauchi, Toshihiko; Nakazawa, Ichiro*
Japanese Journal of Applied Physics, 26(11), p.1933 - 1934, 1987/11
Anti-stokes rotational raman lines in molecular nitrogen gas were used for the calibration of Thomson-scattering apparatus. It was found that molecular nitrogen gas is suitable for a vessel having strong stray lighta. The polarization rario was 0.16 using linear-polarized laser light.
; *
Appl.Opt., 24, p.700 - 709, 1985/00
Times Cited Count:20 Percentile:74.16(Optics)no abstracts in English
; *
Japanese Journal of Applied Physics, 24(11), p.1528 - 1531, 1985/00
Times Cited Count:4 Percentile:31.89(Physics, Applied)no abstracts in English
; *;
Japanese Journal of Applied Physics, 23(10), p.1389 - 1397, 1984/00
Times Cited Count:8 Percentile:48.17(Physics, Applied)no abstracts in English
 ) in the bulk gas phase
) in the bulk gas phaseYokoyama, Keiichi; Yoshida, Fumiko
no journal, ,
Isotope-selective heating, the principle of our new cesium isotope separation scheme, was demonstrated by the stimulated Raman scattering with near infrared femtosecond laser pulses. At the room temperature and 200 Torr of gas pressure, the bulk nitrogen gas was excited with focused Ti:S laser pulses, which was shaped to eight-pulse train from a single 50 fs, 800 nm pulse. The field strength reached 3 10
10 W/cm
W/cm . The pulse interval was set to be 8.4 ps, the classical rotation period of
. The pulse interval was set to be 8.4 ps, the classical rotation period of  N
N . The rotational state distribution was measured by the coherent anti-Stokes Raman scattering spectroscopy. Comparing the results between
. The rotational state distribution was measured by the coherent anti-Stokes Raman scattering spectroscopy. Comparing the results between  N
N and
and 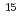 N
N , we can conclude that large displacement of rotational distribution occurs for
, we can conclude that large displacement of rotational distribution occurs for  N
N but not for
but not for 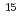 N
N .
.
Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Akiyama, Daisuke*; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*; Kirishima, Akira*
no journal, ,
Since uranium (U) can take numerous chemical states depending on conditions to which it is exposed, the chemical behavior of fuel components is needed for fuel debris treatment generated by Fukushima Daiichi NPS accident. Therefore, understanding on the chemistry of the U materials is essential to estimate these materials' stabilities. One of the important chemical dynamics of uranium is oxidative dissolution of U(IV) materials. It is well known that under the action of ionizing radiation U(IV) oxide matrix of nuclear fuel is gradually oxidized to U(VI) that is fairly water-soluble as uranyl ion. Then, the dissolution of the matrix and the release of radionuclides take place by contact with water. Such chemical degradation may occur for the fuel debris in the Fukushima Daiichi NPS. Hence, we have been investigating the chemical stability of U materials which are anticipated to be included in the fuel debris. As a first step of the investigation, we applied spectroscopic analysis, namely Raman scattering and fluorescence microscopic spectroscopies and Fe-57 M ssbauer spectroscopy for chemical characterization of a pyrochemical product prepared from U(IV) oxide and stainless steel. We have employed these spectroscopic techniques since they are applicable for surface analysis. This is a specific advantage for the investigation of chemical degradation of solid U materials.
ssbauer spectroscopy for chemical characterization of a pyrochemical product prepared from U(IV) oxide and stainless steel. We have employed these spectroscopic techniques since they are applicable for surface analysis. This is a specific advantage for the investigation of chemical degradation of solid U materials.
Suzuki, Seiya
no journal, ,
Suzuki, Seiya; Terasawa, Tomoo; Katsube, Daiki*; Yano, Masahiro; Tsuda, Yasutaka; Yuhara, Junji*; Yoshigoe, Akitaka; Asaoka, Hidehito
no journal, ,
no abstracts in English
Suzuki, Seiya
no journal, ,
no abstracts in English